How Aquatic Life Survives in Winter?
Water’s Strange Properties Keep Life Thriving in Frozen Lakes
How do fish survive when a lake freezes over? Is the whole lake frozen solid, or just the surface? Do they hide in some secret warm spot, or is there something special about the environment itself?
It’s all thanks to a special property of water. You’d be surprised how it makes this possible.
Water, the wonder molecule
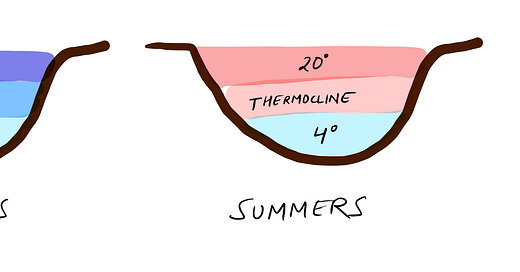
Ice forming on water is pretty amazing when you think about it. Unlike most things, water doesn’t just keep shrinking as it gets colder.
Most liquids behave in a straightforward way when they cool down: they shrink. The colder they get, the slower their molecules move, and the closer they’re pulled together by attractive forces. When they freeze, they shrink even more because solids tend to be tightly packed.
Water, though, is different. As it cools, it contracts like you'd expect—until it hits about 4°C. Below that, it starts expanding sli…
Keep reading with a 7-day free trial
Subscribe to Science Sundays to keep reading this post and get 7 days of free access to the full post archives.